Given the data in the table below, ΔH°rxn for the reaction SO3 (g) + H2O (l) → H2SO4 (l)
Is __________ kJ. 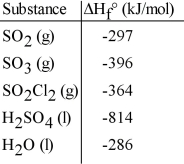
Definitions:
Motorcycle Manufacturers
Companies that design, produce, and market motorcycles and potentially other motorized two-wheeled vehicles.
Equilibrium Quantity
The quantity of goods or services that is supplied and demanded at the equilibrium price, where demand and supply are balanced.
Equilibrium Price
The price at which the quantity of a good or service demanded by consumers equals the quantity supplied by producers, resulting in a market balance.
Demand
The quantity of a good or service that consumers are willing and able to purchase at various prices.
Q22: Which one of the following is a
Q37: Which one of the choices below is
Q39: The correct ground-state electron configuration for silver
Q65: The alkali metal that is used to
Q80: How many grams of CH<sub>3</sub>OH must be
Q114: In which species does sulfur have the
Q142: Lead (II)carbonate decomposes to give lead (II)oxide
Q144: Given the data in the table below,
Q156: The total number of atoms in 0.111
Q163: Lithium and nitrogen react to produce lithium