Given the data in the table below and ΔH°rxn for the reaction SO2Cl2 (g) + 2 H2O (l) → H2SO4 (l) + 2HCl (g) ΔH° = -62 kJ
ΔH°f of HCl (g) is __________ kJ/mol. 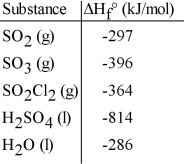
Definitions:
Egos
A person's sense of self-esteem or self-importance.
Corporate Integration
The process of combining different companies or business functions within a corporation to create a more efficient and unified entity.
Joint Mergers
The combination of assets, operations, or management between two or more entities to pursue common goals or improve efficiency.
Client Opposition
Situations where clients or consumers express disagreement or resistance against a company's practices, products, or services.
Q56: Lattice energy is _.<br>A)the energy required to
Q110: All of the group VIA elements are
Q112: Which of the following would require the
Q115: What is the molarity of a NaOH
Q116: Given the following reactions N<sub>2</sub> (g)+ 2
Q133: Of the following elements, _ has the
Q138: Which of the subshells below do not
Q145: Under what condition(s)is the enthalpy change of
Q149: The complete electron configuration of argon, element
Q171: In the generation of most anions, the