Given the data in the table below, ΔH°rxn for the reaction Ca(OH) 2 + 2 H3AsO4 → Ca(H2AsO4) 2 + 2 H2O
Is __________ kJ. 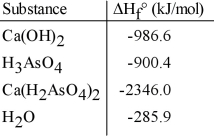
Definitions:
Cost Factor
A numerical figure that represents the cost of a specific element of production or service, used in calculating the total cost.
Financing Receivables
The process of selling accounts receivable to a third party to improve cash flow and reduce risk.
Probability of Nonpayment
The likelihood or risk that a borrower will not be able to make the scheduled payments on their debt obligations.
Revenue
The total income generated by a company from its business activities before any expenses are subtracted.
Q7: Write the balanced equation for the reaction
Q11: The molarity (M)of an aqueous solution containing
Q20: What color of visible light has the
Q60: A sulfur oxide is 50.0% by mass
Q65: The alkali metal that is used to
Q67: What is the maximum number of double
Q104: The value of ΔH° for the reaction
Q117: What is the de Broglie wavelength (m)of
Q127: The molar heat capacity of a compound
Q131: The value of ΔH° for the reaction