Given the data in the table below, ΔH°rxn for the reaction 3NO2 (g) + H2O (l) → 2HNO3 (aq) + NO (g)
Is __________ kJ. 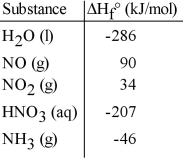
Definitions:
Q5: How many different principal quantum numbers can
Q5: Given that the average bond energies for
Q25: The compound responsible for the characteristic smell
Q27: The formula weight of silver chromate (Ag<sub>2</sub>CrO<sub>4</sub>)is
Q31: The de Broglie wavelength of a bullet
Q81: The electron configuration of the valence electrons
Q81: The formula of palladium(IV)sulfide is _.<br>A)Pd<sub>2</sub>S<sub>4</sub><br>B)PdS<sub>4</sub><br>C)Pd4<sub>S</sub><br>D)PdS<sub>2</sub><br>E)Pd<sub>2</sub>S<sub>2</sub>
Q98: Which solution contains the largest number of
Q100: Of the following statements, _ is not
Q102: A weak electrolyte exists predominantly as _