Multiple Choice
The value of ΔH° for the following reaction is 177.8 kJ. The value of ΔHf° for CaO(s) is __________ kJ/mol. CaCO3 (s) → CaO (s) + CO2 (g) 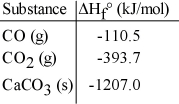
Definitions:
Related Questions
Q2: The atomic radius of iodine is one-half
Q8: The 4d subshell in the ground state
Q18: In which orbital does an electron in
Q30: The molecular weight of acetic acid (
Q50: The net ionic equation for the reaction
Q83: A valid Lewis structure of _ cannot
Q103: The specific heat capacity of lead is
Q144: Given the data in the table below,
Q152: In which reaction does the oxidation number
Q158: How many quantum numbers are necessary to