Given the data in the table below, ΔH°rxn for the reaction Ag2O (s) + H2S (g) → Ag2S (s) + H2O (l)
Is __________ kJ. 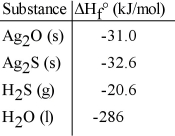
Definitions:
Active Listeners
Individuals who fully concentrate, understand, respond, and then remember what is being said in conversations.
Conforming Goods
Goods that conform to contract specifications.
Right of Refusal
A contractual right that gives an individual or entity the opportunity to enter a business transaction with another party before anyone else can.
Perfect Tender Rule
The requirement that a seller deliver goods in conformity with the contract, down to the last detail.
Q5: Given that the average bond energies for
Q7: Write the balanced equation for the reaction
Q10: Based on the octet rule, phosphorus most
Q19: The element phosphorus exists in two forms
Q21: The value of ΔH° for the following
Q22: Complete and balance the following reaction, given
Q82: The condensed electron configuration of krypton, element
Q99: Iron and chlorine form an ionic compound
Q114: Which of the following is not a
Q170: Silver ions can be precipitated from aqueous