Using Bohr's equation for the energy levels of the electron in the hydrogen atom, determine the 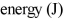 of an electron in the n = 4 level.
of an electron in the n = 4 level.
Definitions:
Larger Culture
The dominant set of norms, values, beliefs, and practices that characterizes a larger society or population group.
Rationalization
The process of making actions or outcomes seem reasonable or logical, often by creating a plausible explanation.
Efficient Means
Methods or strategies that achieve desired outcomes with the least amount of waste, effort, or expense.
Unintended
Outcomes or effects that were not planned or foreseen.
Q16: The value of ΔH° for the reaction
Q49: The energy of a photon that has
Q63: In their compounds, the charges on the
Q94: How many moles of K<sup>+</sup> are present
Q120: Of the following, which gives the correct
Q121: What is the enthalpy change (in kJ)of
Q130: How many moles of magnesium oxide are
Q139: In which reaction does the oxidation number
Q161: The energy of a photon of light
Q177: What is the concentration (M)of sodium ions