Multiple Choice
Using the table of average bond energies below, the ΔH for the reaction is __________ kJ. 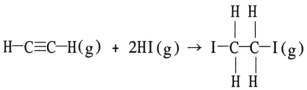 Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
Bond: C≡C C-C H-I C-I C-H D (kJ/mol) : 839 348 299 240 413
Definitions:
Related Questions
Q9: There are _ orbitals in the third
Q25: The de Broglie wavelength of a 6.0
Q26: Bond enthalpy is _.<br>A)always positive<br>B)always negative<br>C)sometimes positive,
Q32: When the electron in a hydrogen atom
Q63: The hybridization of the carbon atom in
Q66: The order of MO energies in B<sub>2</sub>,
Q73: The hybridization of the oxygen atom labeled
Q81: The electron configuration of the valence electrons
Q103: Of the following molecules, only _ is
Q111: The list that correctly indicates the order