Using the table of bond dissociation energies, the ΔH for the following reaction is __________ kJ. 2HCl (g) + F2 (g) → 2HF (g) + Cl2 (g) 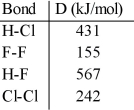
Definitions:
Research Hypothesis
Statement regarding an expected or predicted relationship between variables.
Ordinal Variables
Variables that represent categories with a natural order or ranking, but where the distances between categories are not necessarily equal.
Spearman Rank-Order
A nonparametric measure of rank correlation that assesses how well the relationship between two variables can be described using a monotonic function.
Pearson Correlation
A measure of the linear correlation between two variables X and Y, giving a value between -1 and 1 which indicates the extent of their linear relationship.
Q7: The density of krypton gas at 1.21
Q9: CO (5.00 g)and CO<sub>2</sub> (5.00 g)were placed
Q24: In order to exhibit delocalized π bonding,
Q27: In which of the molecules does the
Q36: The molar volume of a gas at
Q41: A gas at a pressure of 10.0
Q47: The n = 1 shell contains _
Q57: What is the coefficient of H<sub>2</sub>O when
Q110: In comparing the same two atoms bonded
Q145: Zinc reacts with aqueous sulfuric acid to