The reaction below is used to produce methanol:
CO (g)+ 2H2 (g)→ CH3OH (l)ΔHrxn = -128 kJ
(a)Calculate the C-H bond energy given the following data: 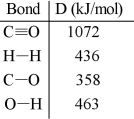 (b)The tabulated value of the (C-H)bond energy is 413 kJ/mol. Explain why there is a difference between the number you have calculated in (a)and the tabulated value.
(b)The tabulated value of the (C-H)bond energy is 413 kJ/mol. Explain why there is a difference between the number you have calculated in (a)and the tabulated value.
Definitions:
Overextension
A language development error where a child applies a word too broadly to objects that do not fit that word's meaning.
Referential Style
A mode of communication primarily concerned with conveying information and facts.
Telegraphic Speech
A stage in language development where young children speak in short, simple combinations of words that convey a clear meaning, resembling telegraphic sentences.
Vocabulary
The set of words that an individual knows and uses, reflecting language development and proficiency.
Q18: A tank containing both HF and HBr
Q52: Which of the following statements is false?<br>A)The
Q61: Screening of the nuclear charge by core
Q62: What is the partial pressure (in mm
Q71: There are _ unpaired electrons in a
Q73: The hybridization of the oxygen atom labeled
Q85: Based on molecular orbital theory, there are
Q102: A gas is considered "ideal" if one
Q129: The molecular weight of a gas is
Q167: The molecular weight of a gas that