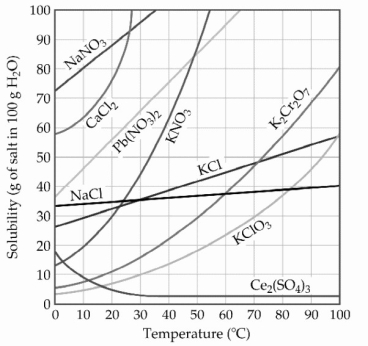
-The solubility of MnSO4 monohydrate in water at 20 °C is 70.0 g per 100.0 mL of water. A solution at 20 °C that is 0.401 M in MnSO4 monohydrate is best described as a(n) ________ solution. The formula weight of MnSO4 monohydrate is 168.97 g/mol.
Definitions:
Manage
To oversee or control the operation, use, or conduct of something or someone, typically in a professional environment.
Hybrid Boards
Governance structures that combine elements of both nonprofit and for-profit boards of directors, typically found in social enterprises.
Elected Members
Individuals chosen through a voting process to hold a particular office or position, typically within governmental or organizational frameworks.
Appointed Members
Individuals chosen or designated to serve on boards, committees, or other bodies based on specific criteria or qualifications rather than elected by a general vote.
Q8: Which of the following cannot be a
Q20: Using the VSEPR model, the molecular geometry
Q22: The equilibrium expression for K<sub>p</sub> for the
Q38: The value of K<sub>eq</sub> for the equilibrium
Q66: The addition of hydrochloric acid and _
Q80: The boiling points of normal hydrocarbons are
Q104: A solution contains 150.8 grams of NaCl
Q104: The concentration of reactants or products at
Q137: Since air is a mixture, it does
Q157: There is/are _ π bond(s)in the molecule