Given the following reaction at equilibrium, if Kc = 1.90 × 1019 at 25.0°C, Kp = __________. H2 (g) + Br2 (g) 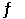 2 HBr (g)
2 HBr (g)
Definitions:
Defective
Refers to a flaw or imperfection in a product or service that makes it unsafe or unsuitable for its intended use.
Dishonors
The refusal or inability of a bank to pay a check or draft when presented for payment.
Insufficient Funds
A situation where an account does not have enough money to cover transactions.
Liable
Liable means responsible by law; legally answerable.
Q3: An elastomer will fail to regain its
Q11: Semiconductors are less conductive than metals because
Q14: An aqueous solution contains 0.150 M HCl
Q20: Which substance in the reaction below either
Q30: Elementary reactions involving the simultaneous collision of
Q52: The rate limiting step in a reaction
Q58: A 7.0 × 10<sup>-3</sup> M aqueous solution
Q64: Compounds found in fossil fuels that contain
Q73: 18 karat gold contains _% gold.<br>A)18<br>B)25<br>C)89<br>D)75<br>E)1.0 x
Q77: In the equation below, M is most