A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction ensues: I2 (g) + Br2 (g) 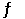 2IBr (g)
2IBr (g)
When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr. The value of Keq is __________.
Definitions:
Net Float
The difference between checks written and deposited in the bank not yet cleared or settled.
Ledger Balance
The total balance in a ledger account at a particular point in time, reflecting all transactions up to that moment.
Available Balance
The amount of funds in a checking or savings account that is accessible for immediate use, taking into account any pending transactions.
Net Collection Float
Net collection float refers to the difference in time between when a check is deposited into a bank account and when the funds are available for use, affecting a company's cash flow.
Q5: Why does the upper atmosphere contain only
Q30: Which of the following expressions is the
Q34: At equilibrium, _.<br>A)all chemical reactions have ceased<br>B)the
Q63: Chromium crystallizes in a body-centered cubic unit
Q73: The magnitudes of K<sub>f</sub> and of K<sub>b</sub>
Q81: Sulfur compounds in the atmosphere are equally
Q89: The solubility of slightly soluble salts containing
Q92: Of the compounds below, the one that
Q122: Determine the pH of a 0.35 M
Q150: Molality is defined as the _.<br>A)moles solute/moles