The equilibrium constant (Kp) for the interconversion of PCl5 and PCl3 is 0.0121: PCl5 (g) 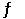 PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)
A vessel is charged with PCl5, giving an initial pressure of 0.123 atm. At equilibrium, the partial pressure of PCl3 is __________ atm.
Definitions:
Media Sharing Sites
Online platforms that allow users to upload, share, and sometimes monetize their multimedia content.
Q34: The acid-dissociation constant, K<sub>a</sub>, for gallic acid
Q42: A solution is prepared by dissolving 7.00
Q44: The average rate of disappearance of A
Q49: A 0.15 M aqueous solution of the
Q58: The mole fraction of helium in dry
Q67: Which compound listed below has the greatest
Q71: Which of the following acids will be
Q78: For a given substance that exhibits liquid-crystalline
Q101: An aqueous solution contains 0.050 M of
Q127: The magnitude of the rate constant is