Given the following reaction at equilibrium, if Kc = 1.90 × 1019 at 25.0°C, Kp = __________. H2 (g) + Br2 (g) 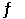 2 HBr (g)
2 HBr (g)
Definitions:
Redeem
The act of exchanging something, such as a coupon or financial securities, for a specific value or service.
Maturity Date
is the specified date on which the final payment of a loan, bond, or other financial instrument is due to be paid.
Face Value
The nominal or dollar value printed on a financial instrument, such as a bond or stock certificate.
Bond Certificate
A physical document representing the investor's right to receive the principal and interest from a bond issuer.
Q20: What is the molar solubility of silver
Q25: Silicon technology is based on the fact
Q36: The K<sub>eq</sub> for the equilibrium below is
Q38: Which of the following is classified as
Q58: A 25.0 mL sample of 0.723 M
Q69: In addition to nitrogen oxides, _ are
Q78: For the reaction aA + Bb →
Q92: A volatile liquid is one that _.<br>A)is
Q106: A _ liquid crystal has the least
Q111: The partial pressure of a component in