The equilibrium constant for the gas phase reaction N2 (g) + 3H2 (g) 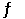 2NH3 (g)
2NH3 (g)
Is Keq = 4.34 × 10-3 at 300°C. At equilibrium, __________.
Definitions:
Experimental
Pertaining to a method of investigation or testing that involves controlled conditions to explore hypotheses, establish causality, or discover effects.
Logical
Pertaining to clear, formal reasoning, or relating to the use of reason and understanding to assess ideas and arguments.
Intuitive Thinking
Intuitive thinking is a cognitive process that occurs without explicit reasoning, relying on instinctive feelings rather than systematic analysis of information.
Rational
Based on or in accordance with reason or logic.
Q6: The majority of ozone that protects against
Q9: What is the predominant intermolecular force in
Q20: What is the molar solubility of silver
Q28: Exactly 3.5 moles if N<sub>2</sub>O<sub>4</sub> is placed
Q40: Of the following, only _ does not
Q81: Acetic acid is a weak acid that
Q90: Calculate the pOH of 0.716 M anilinium
Q93: For a dilute aqueous solution, a concentration
Q103: What is the molarity of sodium chloride
Q128: Which energy difference in the energy profile