Multiple Choice
The equilibrium constant for the gas phase reaction 2NH3 (g) 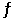 N2 (g) + 3H2 (g)
N2 (g) + 3H2 (g)
Is Keq = 230 at 300°C. At equilibrium, __________.
Definitions:
Related Questions
Q8: For a reaction to be spontaneous under
Q12: The rate constant for a particular second-order
Q48: The most likely van't Hoff factor for
Q58: The freezing point of ethanol (C<sub>2</sub>H<sub>5</sub>OH)is -114.6°C.
Q60: A solution of acetic acid is 2.0%
Q73: Calculate the pOH of a 0.0627 M
Q84: Which of the following has dispersion forces
Q91: The "scale" caused by hard water is
Q120: How many moles of B are present
Q123: The concentration of urea (MW = 60.0