Consider the following reaction at equilibrium: 2NH3 (g) 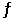 N2 (g) + 3H2 (g) ΔH° = +92.4 kJ
N2 (g) + 3H2 (g) ΔH° = +92.4 kJ
Le Châtelier's principle predicts that adding N2 (g) to the system at equilibrium will result in __________.
Definitions:
Carbocation Rearrangement
A process where a carbocation (a positively charged carbon atom) changes its structure by moving a hydrogen atom or a hydrocarbon group to a different carbon atom, often leading to a more stable carbocation.
Aqueous Acid
An acid solution in which water is the solvent, often used in chemical reactions that require a proton source.
Stereochemical
Pertaining to the spatial arrangement of atoms in a molecule and the impact of this arrangement on its chemical behavior.
Regio-
A prefix in chemistry indicating the region or specific location in a molecule where a reaction takes place or a particular group is located.
Q10: The concentration of fluoride ions in a
Q24: A result of the common-ion effect is
Q42: Molecules with only single bonds do not
Q43: At 20°C, a 2.32 M aqueous solution
Q51: Which one of the following vitamins is
Q52: Which one of the following is a
Q59: The formula weight of FeCl<sub>3</sub><sup>.</sup>6H<sub>2</sub>O is _.
Q61: 24 karat gold contains _% gold.<br>A)24<br>B)25<br>C)5.0 x
Q78: Calculate the pH of a 0.100 M
Q105: Of the following, the entropy of gaseous