Consider the following reaction at equilibrium: 2CO2 (g) 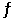 2CO (g) + O2 (g) ΔH° = -514 kJ
2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that adding O2 (g) to the reaction container will __________.
Definitions:
Psychosexual Stage
Periods in Sigmund Freud's theory during which a child's pleasure-seeking energies focus on specific areas of the body leading to personality development.
Displacement
A defense mechanism where emotional impulses are redirected from the original source to a non-threatening target.
Aggressive Impulses
Innate drives or tendencies to act or react in a hostile or violent manner towards others or oneself.
Regression
A defense mechanism leading individuals to revert to earlier stages of development and behaviors under stress or discomfort.
Q2: A solution with a solute concentration greater
Q7: The phase diagram of a substance is
Q16: Calculate the percent ionization of formic acid
Q43: Nitric acid is a strong acid. This
Q47: For the endothermic reaction CaCO<sub>3</sub> (s) <img
Q50: The substance with the largest heat of
Q56: The average rate of disappearance of I<sup>-</sup>
Q93: _ are particularly polarizable.<br>A)Small nonpolar molecules<br>B)Small polar
Q118: The conjugate acid of HSO<sub>4</sub><sup>-</sup> is _.<br>A)SO<sub>4</sub><sup>2-</sup><br>B)H<sub>2</sub>SO<sub>4</sub><br>C)HSO<sub>4</sub><sup>+</sup><br>D)H<sup>+</sup><br>E)HSO<sub>3</sub><sup>+</sup>
Q129: The molarity of urea in a solution