Consider the following reaction at equilibrium: 2CO2 (g) 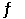 2CO (g) + O2 (g) ΔH° = -514 kJ
2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that an increase in temperature will __________.
Definitions:
Current Income Statement
A financial report showing a company's revenues, expenses, and net income over a specific period, typically a quarter or year.
Prior Year's Income Statement
A financial report that shows a company's financial performance in terms of revenue, expenses, and net income for the previous fiscal year.
Statement of Cash Flows Worksheet
A financial document used to analyze and prepare the statement of cash flows, showing how cash changes from operating, investing, and financing activities.
Significant Noncash
Transactions that have a major impact on a company's financial statements but do not involve cash flow.
Q2: A possible mechanism for the overall reaction
Q8: Which of the following is not classified
Q39: Pure _ and pure _ are excluded
Q55: The solubility of manganese (II)hydroxide (Mn(OH)<sub>2</sub>)is 2.2
Q61: A solution is prepared by dissolving 23.7
Q92: The K<sub>a</sub> of benzoic acid is 6.30
Q111: The pH of a 0.60 M aqueous
Q122: On a clear day at sea level,
Q131: Using the data in the table, which
Q150: Molality is defined as the _.<br>A)moles solute/moles