Consider the following reaction at equilibrium: C (s) + H2O (g) 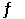 CO (g) + H2 (g)
CO (g) + H2 (g)
Which of the following conditions will increase the partial pressure of CO?
Definitions:
Women
Female adults, recognized for their roles and contributions across various aspects of society.
Men
Adult human males, who have distinct physiological and gender-related characteristics and health concerns compared to women.
Health Psychology
A field focused on how psychological, behavioral, and cultural factors contribute to physical health and illness.
Gerontology
The study of the aging process and the challenges encountered as people grow older.
Q7: The equilibrium-constant expression for the reaction Ti
Q14: The value of ΔG° at 25°C for
Q22: The process of adding controlled amounts of
Q26: Of the reactions involved in the photodecomposition
Q30: A sample of natural rubber (200.0 g)is
Q51: Calculate the concentration (in M)of hydronium ions
Q59: On the phase diagram shown above, the
Q75: The overall order of a reaction is
Q83: In which of the following aqueous solutions
Q88: Of the following, _ should have the