Consider the following reaction at equilibrium. 2CO2 (g) 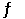 2CO (g) + O2 (g) ΔH° = -514 kJ
2CO (g) + O2 (g) ΔH° = -514 kJ
Le Châtelier's principle predicts that the equilibrium partial pressure of CO (g) can be maximized by carrying out the reaction __________.
Definitions:
Willingness
The state of being prepared to do something; readiness or inclination to engage in an activity or process.
Behaviors
Observations of individual actions and responses, often studied in psychology to understand and predict human interactions.
Values
Core principles or beliefs that guide behavior and decision-making.
Individuals
Separate persons considered separately from a group or collectively.
Q6: Using the data in the table, which
Q7: If a rate law is second order
Q13: Given the following table of thermodynamic data,
Q24: What is the pH of a sodium
Q43: The extent of ionization of a weak
Q47: The mole fraction of carbon dioxide in
Q56: Most fresh water in the U.S. is
Q63: A reversible process is one that _.<br>A)can
Q65: Based on the following information, which compound
Q94: Which one of the following statements regarding