Dinitrogentetraoxide partially decomposes according to the following equilibrium: N2O4 (g) 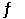 2NO2 (g)
2NO2 (g)
A 1.00-L flask is charged with 0.0400 mol of N2O4. At equilibrium at 373 K, 0.0055 mol of N2O4 remains. Keq for this reaction is __________.
Definitions:
Machine-Hours
The total time that machinery is operating and engaged in production activities during a specified period.
Job T687
A specific job or project identifier used within a job costing system to track costs and progress.
Unit Product Cost
The sum of variable and fixed expenses incurred in the creation of a single product unit.
Direct Labor-Hours
The total hours of labor directly involved in manufacturing a product or providing a service.
Q13: Given the following table of thermodynamic data,
Q34: A solution contains 11% by mass of
Q38: Which of the following liquids will have
Q42: The value of ΔG° at 25°C for
Q46: The pH of a 0.15 M aqueous
Q64: The transition metals in group _ have
Q85: Which compound has the strongest intermolecular forces?<br>A)CBr<sub>4</sub><br>B)C<sub>12</sub>H<sub>26</sub><br>C)CI<sub>4</sub><br>D)N<sub>2</sub><br>E)O<sub>2</sub>
Q96: The concentration (M)of HCl in a solution
Q113: Compounds composed of a salt and water
Q116: The principal source of the difference in