Given the following reaction: CO (g) + 2H2(g) 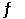 CH3OH (g)
CH3OH (g)
In an experiment, 0.42 mol of CO and 0.42 mol of H2 were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.29 mol of CO remaining. Keq at the temperature of the experiment is __________.
Definitions:
Accounts Receivable Turnover
A financial ratio indicating how many times a company collects its average accounts receivable balance in a given period.
Year 2
Designates the second fiscal or calendar year in a sequence of years, crucial for tracking progress, budgets, and planning in various contexts.
Average Collection Period
The average amount of time it takes for a business to receive payments owed by its customers for goods or services sold on credit.
Year 2
Refers to the second year of a project, company's fiscal year, or any specified time period.
Q11: Semiconductors are less conductive than metals because
Q14: Of the following, only _ is not
Q21: Components of Air Mole Fraction Nitrogen 0.781<br>Oxygen
Q28: The pH of a 0.55 M aqueous
Q43: The extent of ionization of a weak
Q59: On the phase diagram shown above, the
Q61: The instantaneous rate of a reaction can
Q69: In addition to nitrogen oxides, _ are
Q84: Using the data in the table, which
Q139: A supersaturated solution _.<br>A)is one with more