A sealed 1.0 L flask is charged with 0.500 mol of I2 and 0.500 mol of Br2. An equilibrium reaction ensues: I2 (g) + Br2 (g) 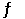 2IBr (g)
2IBr (g)
When the container contents achieve equilibrium, the flask contains 0.84 mol of IBr. The value of Keq is __________.
Definitions:
Q2: Vulcanization involves heating rubber with sulfur dioxide
Q8: Which of the following is not classified
Q10: The enzyme nitrogenase converts _ into _.<br>A)ammonia,
Q16: Of the following, _ is the most
Q17: What compound in limestone and marble is
Q69: Which of the following is most likely
Q75: For any buffer system, the buffer capacity
Q75: The dissolution of water in octane (C<sub>8</sub>H<sub>18</sub>)is
Q103: What is meant by the salinity of
Q132: K<sub>a</sub> for HF is 7.0 × 10<sup>-4</sup>.