At 200°C, the equilibrium constant (Kp) for the reaction below is 2.40 × 103. 2NO (g) 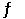 N2 (g) + O2 (g)
N2 (g) + O2 (g)
A closed vessel is charged with 36.1 atm of NO. At equilibrium, the partial pressure of O2 is __________ atm.
Definitions:
Adversely Affected
Negatively impacted or harmed by a particular event, action, or decision.
Behavioural Disinhibition
A personality trait describing an inability to inhibit behavioural impulses, rebelliousness, aggressiveness, and risk-taking that are associated with the development of alcohol problems.
Personality Characteristic
Traits or qualities that contribute to an individual's distinctive patterns of thinking, feeling, and behaving.
Behavioural Tolerance
Through the principles of classical conditioning, cues in the environment can become conditioned stimuli to the effects of drug use. These cues cause the individual to anticipate the drug effects so that when the drug is actually administered the effects are diminished. Tolerance, or the need for a greater amount of drug for the same effect, is greatest when the conditioned environmental cues are present.
Q19: Select the substance that is thought to
Q23: Consider the following chemical reaction: H<sub>2</sub> (g)+
Q36: How many milliliters of 0.120 M NaOH
Q37: What is the molar solubility of silver
Q37: Steel is<br>A)an alloy of iron.<br>B)pure iron.<br>C)oxidized iron.<br>D)a
Q52: The rate limiting step in a reaction
Q61: ΔS is positive for the reaction _.<br>A)2
Q91: The "scale" caused by hard water is
Q103: What is the molarity of sodium chloride
Q112: The heat of fusion of water is