Multiple Choice
Given the following reaction at equilibrium at 450.0°C: CaCO3 (s) 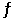 CaO (s) + CO2 (g)
CaO (s) + CO2 (g)
If pCO2 = 0.0135 atm, Kc = __________.
Definitions:
Related Questions
Q8: Which of the following is not classified
Q12: The entropy change accompanying any process is
Q34: The acid-dissociation constant, K<sub>a</sub>, for gallic acid
Q46: The pH of a 0.15 M aqueous
Q47: The mole fraction of carbon dioxide in
Q56: The K<sub>eq</sub> for the equilibrium below is
Q94: The Procter & Gamble Company product called
Q104: Which gaseous sulfur compound combines with water
Q108: The C-O bond dissociation energy in CO<sub>2</sub>
Q112: The concentration of S<sub>2</sub>O<sub>8</sub><sup>2-</sup> remaining at 1600