Given the following reaction at equilibrium, if Kp = 0.990 at 250.0°C, Kc = __________. PCl5 (g) 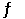 PCl3 (g) + Cl2 (g)
PCl3 (g) + Cl2 (g)
Definitions:
Electrocardiography
A medical test that measures the electrical activity of the heart to identify various heart conditions.
Stylus
A tool or device designed to input commands or write on a touchscreen display or graphics tablet.
Sketch
A rapidly executed freehand drawing that is not intended as a finished work, often used to visualize ideas.
Lead Arrangement
Refers to the specific configuration and placement of electrodes in electrocardiography for monitoring the electrical activity of the heart.
Q4: The solubility of Ar in water at
Q9: If ΔG° for a reaction is greater
Q22: Calculate the maximum concentration (in M)of silver
Q23: As the concentration of a solute in
Q42: The K<sub>eq</sub> for the equilibrium below is
Q58: The value of K<sub>eq</sub> for the following
Q60: The value of ΔH° for the formation
Q70: The amount of atomic O relative to
Q110: An aqueous solution contains 0.050 M of
Q127: Calculate the molality of a 10.0% (by