Phosphorous Trichloride and Phosphorous Pentachloride Equilibrate in the Presence of Molecular
Phosphorous trichloride and phosphorous pentachloride equilibrate in the presence of molecular chlorine according to the reaction: PCl3 (g) + Cl2 (g) 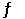 PCl5 (g)
PCl5 (g)
An equilibrium mixture at 450 K contains
PPCl3 = 0.124 atm,
PCl2 = 0.157 atm, and
PPCl5 = 1.30 atm. What is the value of Kp at this temperature?
Definitions:
Q3: Calculate the mole fraction of phosphoric acid
Q7: If a rate law is second order
Q8: The K<sub>eq</sub> for the equilibrium below is
Q34: The principal component of smog is sulfur
Q38: The value of ΔG° for a reaction
Q88: A solution is prepared by dissolving 0.60
Q92: The conjugate acid of CH<sub>3</sub>NH<sub>2</sub> is _.<br>A)CH<sub>3</sub>NH<sub>2</sub><br>B)CH<sub>3</sub>NH<sub>3</sub><sup>+</sup><br>C)CH3NH<sub>3</sub><sup>+</sup><br>D)CH<sub>3</sub>NH<sup>+</sup><br>E)none
Q100: Calculate the molarity of a 25.4% (by
Q129: Classify the following compounds as weak acids
Q134: The concentration of nitrate ion in a