At 900.0 K, the equilibrium constant (Kp) for the following reaction is 0.345. 2SO2 + O2 (g) 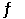 2SO3 (g)
2SO3 (g)
At equilibrium, the partial pressure of SO2 is 35.0 atm and that of O2 is 15.9 atm. The partial pressure of SO3 is __________ atm.
Definitions:
Symbols
Signs or objects used to represent ideas, concepts, or other abstractions.
Script
A sequence of behaviors, actions, or events that are expected to follow a particular order in specific social or situational contexts.
Pajamas
A set of clothing, typically comfortable and loose-fitting, intended for sleep or home use.
Verbal Reports
Accounts or descriptions given orally or in written form, often used to convey observations, experiences, or research findings.
Q2: A solution with a solute concentration greater
Q23: Natural rubber is too soft and chemically
Q52: Calculate the maximum concentration (in M)of magnesium
Q53: Blue LEDs are usually made of _.<br>A)GaAs<br>B)GaP<br>C)GaO<br>D)GaS<br>E)GaN
Q67: The equilibrium expression for K<sub>p</sub> for the
Q94: What is the pH of a buffer
Q102: A chemical bond rupture resulting from the
Q120: The value of ΔS° for the decomposition
Q122: Reaction rates are affected by reactant concentrations
Q149: The solubility of oxygen gas in water