In the coal-gasification process, carbon monoxide is converted to carbon dioxide via the following reaction: CO (g) + H2O (g) 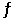 CO2 (g) + H2 (g)
CO2 (g) + H2 (g)
In an experiment, 0.35 mol of CO and 0.40 mol of H2O were placed in a 1.00-L reaction vessel. At equilibrium, there were 0.16 mol of CO remaining. Keq at the temperature of the experiment is __________.
Definitions:
Statement of Income
A financial document that reports a company's financial performance over a specific period, detailing revenues, expenses, and net profit or loss.
Long-Term Investments
Assets that a company intends to hold for more than one year, including stocks, bonds, or real estate.
Bonds
A fixed-income investment representing a loan made by an investor to a borrower, usually corporate or governmental.
Trading Investments
Securities bought and held primarily for sale in the near term to generate income on short-term price differences.
Q3: Calculate the mole fraction of phosphoric acid
Q8: For a reaction to be spontaneous under
Q10: K<sub>b</sub> for NH<sub>3</sub> is 1.8 × 10<sup>-5</sup>.
Q17: What compound in limestone and marble is
Q31: In the _ liquid crystalline phase, the
Q45: The expression for K<sub>p</sub> for the reaction
Q56: Most fresh water in the U.S. is
Q67: The value of ΔS° for the decomposition
Q81: The decomposition of N<sub>2</sub>O<sub>5</sub> in solution in
Q87: The active site of nitrogenase is a