Consider the reaction: FeO (s) + Fe (s) + O2 (g) → Fe2O3 (s)
Given the following table of thermodynamic data, 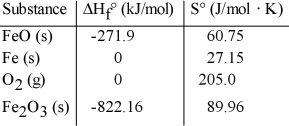 determine the temperature (in °C) above which the reaction is nonspontaneous.
determine the temperature (in °C) above which the reaction is nonspontaneous.
Definitions:
Strategic Change
A systematic approach to transforming an organization's goals, processes, or technologies to address evolving external and internal forces and improve its performance and competitiveness.
Highly Analytical
The ability to critically assess information or situations, often involving detailed examination of components or structure.
Incremental
Describes a process of adding or making changes in small, manageable amounts rather than in one large, comprehensive step.
Momentum For Change
The driving force or energy behind movements or developments aimed at transforming or amending current systems, practices, or beliefs.
Q9: If ΔG° for a reaction is greater
Q11: The K<sub>eq</sub> for the equilibrium below is
Q21: A reversible change produces the maximum amount
Q26: A 25.0 mL sample of 0.150 M
Q34: The electrode at which oxidation occurs is
Q61: ΔS is positive for the reaction _.<br>A)2
Q73: Calculate the pOH of a 0.0627 M
Q76: Disadvantages of the methanol fuel cell compared
Q79: Consider a solution containing 0.100 M fluoride
Q174: The addition of solid potassium cyanide to