Given the thermodynamic data in the table below, calculate the equilibrium constant (at 298 K) for the reaction: 2SO2 (g) + O2 (g) 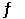 2SO3 (g)
2SO3 (g) 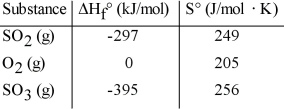
Definitions:
Profits And Losses
The financial gains earned or losses incurred by a business over a specified period.
Economic Model
A simplified representation of economic processes, used to predict and analyze real-world economic behaviors.
Consumer Tastes
Preferences and inclinations of consumers regarding various goods and services, which can influence their purchasing decisions.
Entrepreneurial Decision-Making
The process through which entrepreneurs assess, decide, and act upon business opportunities and ideas.
Q34: What radioactive element is used to diagnose
Q43: What are the products of the reaction
Q48: What is the coefficient of the permanganate
Q51: Of the non radioactive halogens which is
Q52: In a discussion of oxygen compounds, a
Q66: A 0.0035 M aqueous solution of a
Q81: The pH of a solution prepared by
Q84: The pH of a solution prepared by
Q121: This reaction is an example of _.
Q171: The electrical conductivity of _ is low