The equilibrium constant for the following reaction is 3.5 × 108 at 25°C. N2 (g) + 3H2 (g) 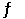 2NH3 (g)
2NH3 (g)
The value of ΔG° for this reaction is __________ kJ/mol.
Definitions:
Total Revenue
refers to the full amount of income generated by the sale of goods or services before any expenses are subtracted.
Sinking Fund
A savings account or fund dedicated to paying off debt or a bond, where money is set aside over time to manage future repayment obligations.
Depreciating Equipment
Equipment or machinery that decreases in value over time due to use, wear and tear, or obsolescence.
Technological Innovation
The development and application of new technologies and ideas to improve goods, services, or processes, enhancing efficiency or value.
Q24: The value of ΔG° at 373 K
Q25: The danger from mixing ammonia with bleach
Q28: The value of ΔH° for the decomposition
Q39: The pH of a solution prepared by
Q43: The main ionic constituent of sea water
Q50: Given the following table of thermodynamic data,
Q88: The production of dead and decaying plant
Q101: The most difficult species to reduce and
Q117: Diamagmetic solids _.<br>A)have atoms with one or
Q142: When two atoms of <sup>2</sup>H are fused