In balancing the nuclear reaction 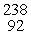 U →
U → 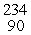 E +
E + 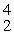 He, the identity of element E is __________.
He, the identity of element E is __________.
Definitions:
Neutrons
Neutral particles found in the nucleus of an atom, contributing to the atom's mass but not its charge.
Protons
Positively charged subatomic particles found in the nucleus of an atom.
Amu
Atomic Mass Unit, a unit of mass used to express atomic and molecular weights, equivalent to one twelfth of the mass of a carbon-12 atom.
Valence Shells
The outermost shell of electrons in an atom, which determines how the atom interacts chemically with other atoms.
Q10: The value of ΔG° at 25°C for
Q13: Given the following table of thermodynamic data,
Q16: The anode of the alkaline battery is
Q49: The combustion of ethene in the presence
Q81: Sulfur compounds in the atmosphere are equally
Q82: How many isomers are possible for C<sub>4</sub>H<sub>10</sub>?<br>A)1<br>B)2<br>C)3<br>D)4<br>E)10
Q84: The normal boiling point of ethanol (C<sub>2</sub>H<sub>5</sub>OH)is
Q135: Of the following, which is most likely
Q155: The oxidation state of silicon in SiO<sub>2</sub><sup>4-</sup>
Q183: The number of electrons in the valence