The class of compound formed in the reaction of the following two substance is a(n)__________. 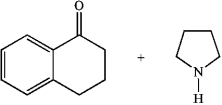
Definitions:
Triple Bond
A type of chemical linkage where two atoms share three pairs of electrons, resulting in a bond that is shorter and stronger than those involving only one or two pairs of electrons.
2-Hexyne
An organic compound with the chemical formula C6H10, consisting of a six-carbon chain with a triple bond between the second and third carbon atoms.
Lewis Line Structure
A diagram representing the valence electrons of atoms within a molecule using dots and lines for bonding pairs.
Line Structure
A simplified representation of a chemical structure, using lines to represent chemical bonds and showing the arrangement of atoms within the molecule.
Q18: What type of intermediate is formed in
Q26: The NMR data would seem to indicate
Q27: What is the major organic product obtained
Q41: _ is a sugar which yields a
Q46: Which carbon in the following carbohydrate is
Q58: What is the IUPAC name of the
Q62: Which of the following gives rise to
Q67: In the following compound, the most acidic
Q77: What is the major organic product obtained
Q102: The nucleophile in the reaction is _.<br>A)A<br>B)B<br>C)C<br>D)D