Given an activation energy of 15 kcal/mol, use the Arrhenius equation to estimate how much faster the reaction will occur if the temperature is increased from 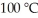 to
to 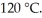 R = 1.987 cal/mol ∙ K.
R = 1.987 cal/mol ∙ K.
Definitions:
Employee Participation
A workplace practice where employees are involved in decision-making processes, potentially improving satisfaction and productivity.
Phantom Stock Plan
A type of employee benefit plan that gives selected employees many of the benefits of stock ownership without giving them any company stock, often tied to the performance or value of the company's stock.
Company Stock
Shares of ownership in a corporation, representing a claim on part of the corporation's assets and earnings.
Performance-Based Pay
A compensation system where an employee's pay is directly tied to their performance metrics, aimed at incentivizing high levels of job performance.
Q1: Which of the following statements correctly describes
Q8: Which of the following terms best describes
Q10: In each of the pairs below, which
Q43: Give the formal charge on nitrogen in
Q46: Which of the following is/are the major
Q62: Provide the major organic product(s)in the reaction
Q68: A stakeholder is any person or entity:<br>A)owning
Q74: Which of the following is the final
Q74: Identify the compound with the highest bond
Q99: Which of the following compounds has an