Consider the following monobromination reaction, then answer the following questions. 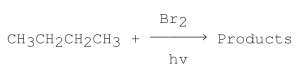
a) Give the structures and the IUPAC names for the products.
b) Give the common names for the products.
c) Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:  d) Calculate the percent yield for each product. (relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
d) Calculate the percent yield for each product. (relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e) Propose a step-by-step mechanism for the major product only.
f) Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g) Does the transition state of the RDS resemble more closely the reactants or the products?
h) Would the value of the activation energy be different for different alkanes? Explain.
i) Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
Definitions:
Generalist Intervention Model
A social work practice model that provides a framework for intervention involving assessment, planning, implementation, evaluation, and advocacy.
Accountability
The obligation of an individual, group, or organization to account for its activities, accept responsibility for them, and to disclose the results in a transparent manner.
Effective Interventions
Actions or strategies implemented to bring about positive change in a situation, behavior, or condition, based on evidence or best practices.
Generalist Practitioner
A social work professional who applies a broad range of skills and techniques to support individuals, families, groups, communities, and systems across various environments and situations.
Q11: Deduce the identity of the following compound
Q43: Provide the systematic name of the compound
Q44: Give the best product(s)for the reaction. <img
Q48: What are the major products from the
Q63: Starting with isopropyl alcohol as the only
Q64: What is the major product of the
Q92: When 1-bromo-2,2-dimethylcyclopentane is heated in ethanol, one
Q107: A compound gave a signal at 203
Q107: Give the best product for the following
Q111: Which of the following bromides reacts readily