Consider the following monobromination reaction, then answer the following questions. 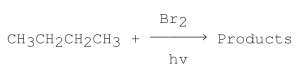
a) Give the structures and the IUPAC names for the products.
b) Give the common names for the products.
c) Calculate ΔH° for the overall reaction using the following data for the indicated bond dissociation energies:  d) Calculate the percent yield for each product. (relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
d) Calculate the percent yield for each product. (relative rate of abstraction of 3° hydrogen is 1600; 2° is 82; and 1° is 1.)
e) Propose a step-by-step mechanism for the major product only.
f) Draw a schematic potential energy diagram for the rate-determining step (RDS)only.
g) Does the transition state of the RDS resemble more closely the reactants or the products?
h) Would the value of the activation energy be different for different alkanes? Explain.
i) Would the reaction slow down or speed up if I2 is used instead of Br2? Explain.
Definitions:
Beginning Inventory
The value of a company’s inventory at the start of an accounting period, carried over from the end of the previous period.
Periodic Inventory System
An accounting method where inventory and cost of goods sold are determined at the end of a period through a physical count.
FIFO Inventory
A method of inventory valuation where the first items produced or acquired are the first ones sold, FIFO stands for "First In, First Out."
Cost of Goods Sold
The total cost of all raw materials, labor, and overhead expenses incurred to produced goods that were sold during a particular period.
Q3: What is the most reactive alkyl halide
Q8: Give the product(s)for the reaction of (R)-2-bromopentane
Q12: Provide the major organic product of the
Q16: Which base, ammonia (NH<sub>3</sub>)or triethylamine [(CH<sub>3</sub>CH<sub>2</sub>)<sub>3</sub>N], would
Q25: Provide the major organic product of the
Q44: Which of the following molecular changes is
Q53: Which of the following protons gives an
Q57: Provide the structure of the major organic
Q106: How many distinct carbon signals are expected
Q118: Rank the following dienes in order of