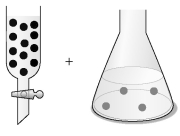
-The concentration of an aqueous solution of Fe2+ can be determined by a redox titration with aqueous bromate ion,BrO3⁻: 6 Fe2+ (aq) + BrO3⁻ (aq) + 6 H⁺ (aq) → 6 Fe3+ (aq) + Br⁻ (aq) + 3 H2O (l)
Assume that the black spheres in the buret represent BrO3⁻ ions,the gray spheres in the flask represent Fe2+ ions,the concentration of the BrO3⁻ ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the Fe2+ in the flask,and what fraction of the BrO3⁻ solution in the buret must be added to the flask to react with all the Fe2+ ions?
Definitions:
Psychology
Scientific study of behavior and mental processes.
Memory
The mental capacity for storing and retrieving information.
Short-term Memory
Retaining a small volume of information actively on hand in the brain for a quick moment.
Forgetting
The loss or inability to recall information that was previously encoded into long-term memory.
Q16: Assign formal charges to each atom in
Q18: What is the bond order of the
Q22: The algebraic signs (+ and -)sometimes written
Q26: Elements that can accommodate more than eight
Q49: Balance the chemical equation given below,and determine
Q116: Of the following,which element has the highest
Q116: What is the mass of 9.00 ×
Q119: Which period 3 element has successive first
Q139: Balance the chemical equation given below,and calculate
Q161: What are the possible values of n