Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 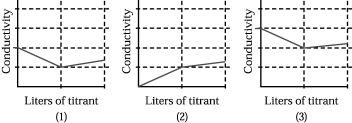
-Which of the above graphs corresponds to titration II?
Definitions:
Enclosures
Materials that are included in the same envelope as the primary letter.
Mixed Punctuation
In writing and typing, the use of both open (starting with an open mark, like a quotation) and closed punctuation styles, involving a mix of necessary punctuation marks.
Complimentary Closing
A polite phrase used to conclude a letter or message before the sender's signature, such as "Sincerely" or "Best regards".
Signature Block
The writer’s name and business title found four lines below the complimentary closing in a business letter.
Q16: The chemical formula for the carbonate ion
Q56: In the reaction of sodium metal with
Q60: An element Y,has the following ionization energies
Q68: Which liberates the most energy?<br>A)Br(g)+ e⁻ →
Q86: How many grams of calcium chloride are
Q88: The electronegativity for both sulfur and carbon
Q99: Which grouping of elements,indicated by letter on
Q107: How many milliliters of 0.300 M Li<sub>2</sub>S
Q133: The element antimony has an atomic weight
Q162: Which one of the following compounds contains