Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 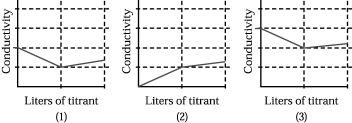
-Which of the above graphs corresponds to titration III?
Definitions:
Volute
A spiral, scroll-like form characteristic of the ancient Greek Ionic and the Roman Composite capital.
Ionic
One of the two systems (or orders) invented in ancient Greece for articulating the three units of the elevation of a classical building: the platform, the colonnade, and the superstructure (entablature). The Ionic order is characterized by, among other features, volutes, capitals, columns with bases, and an uninterrupted frieze. See also Doric.
Greek Column Capital
The topmost element of a column in Greek architecture, varying in design - Doric, Ionic, or Corinthian - and serving both structural and decorative purposes.
Athenian White-Ground Lekythos
A type of ancient Greek vase, often used for storing oil, distinguished by its white background and detailed figural decoration.
Q3: The electronegativity is 2.1 for H and
Q20: The group 4A element that always obeys
Q54: Combustion analysis of 2.796 g of an
Q70: How many lone pairs are on the
Q72: Atoms of which element,indicated by letter on
Q78: The chlorine atom in Cl<sub>2</sub> would be
Q92: The element Al can be found in
Q153: What is the sum of the coefficients
Q166: What is the concentration of HCl in
Q273: _ is a nonmetal that is a