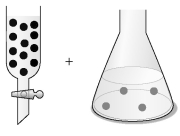
-The concentration of an aqueous solution of Fe2+ can be determined by a redox titration with aqueous bromate ion,BrO3⁻: 6 Fe2+ (aq) + BrO3⁻ (aq) + 6 H⁺ (aq) → 6 Fe3+ (aq) + Br⁻ (aq) + 3 H2O (l)
Assume that the black spheres in the buret represent BrO3⁻ ions,the gray spheres in the flask represent Fe2+ ions,the concentration of the BrO3⁻ ions in the buret is 0.120 M,and the volumes in the buret and the flask are identical.What is the concentration of the Fe2+ in the flask,and what fraction of the BrO3⁻ solution in the buret must be added to the flask to react with all the Fe2+ ions?
Definitions:
Pacemaker
A device implanted in the chest to regulate the heartbeat.
Atrioventricular Node
A part of the cardiac conduction system that coordinates the top heart chambers (atria) contraction with the bottom chambers (ventricles) ensuring a synchronized heartbeat.
Lymph Tissue
Specialized connective tissue that contains large numbers of lymphocytes and is involved in the immune response and the maintenance of bodily fluids levels.
Oral Cavity
The mouth, or buccal cavity, which is the first part of the digestive system and where the mechanical breakdown of food begins.
Q13: How many double and single bonds are
Q14: Of the following elements,which has the lowest
Q26: 2-Propanol has the molecular formula C<sub>3</sub>H<sub>8</sub>O.Which ball
Q105: How many grams of AgNO<sub>3</sub> are needed
Q117: How many mL of a 0.350 M
Q132: The following diagram represents the reaction of
Q195: Three atoms have the following properties. <img
Q200: Which of the following represent isotopes? A:
Q223: Which is most likely to form a
Q239: The charge to mass ratio of an