Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 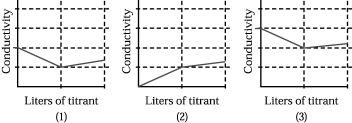
-Which of the above graphs corresponds to titration II?
Definitions:
Employment Eligibility Verification Form I-9
The Employment Eligibility Verification Form I-9 is a U.S. government document used by employers to verify an employee's identity and legal authorization to work in the United States.
Immigration Compliance
The adherence to laws and regulations governing the entry, residence, and work status of non-nationals in a country.
Federal Law
The body of law created by the federal government of a country, encompassing statutes, regulations, and case law.
Medical Insurance Protection
Coverage offered by insurance policies to help cover the cost of medical and surgical expenses of the insured individual.
Q9: Which group of elements,indicated by letter on
Q18: The chemical formula for chlorous acid is<br>A)H<sub>
Q34: How many sulfate ions are there in
Q45: The chemical formula for magnesium nitride is<br>A)Mg(NO<sub>3</sub>)<sub>2</sub>.<br>B)Mg(NO<sub>2</sub>)<sub>2</sub>.<br>C)Mg<sub>3</sub>N<sub>2</sub>.<br>D)MgN<sub>2</sub>.
Q47: The reaction Na<sub>3</sub>PO<sub>4</sub>(aq)+ 3 AgNO<sub>3</sub>(aq)→ Ag<sub>3</sub>PO<sub>4</sub>(s)+ 3
Q62: Which of the following most likely represent
Q86: For the fourth-shell orbital shown below,what are
Q95: What is the ground-state valence-shell electron configuration
Q121: Of the following,which element has the highest
Q157: Ozone is unstable,decomposing to oxygen,as shown in