Assume that the conductivity of a solution depends only on the total concentration of dissolved ions and that you measure the conductivity of three different solutions while performing titrations in which
I.50.00 mL of 0.100 M aqueous CH3CO2H is titrated by addition of 0.100 M NaOH.
II.50.00 mL of 0.100 M aqueous NaBr is titrated by addition of 0.100 M AgNO3.
III.50.00 mL of 0.100 M aqueous CaCl2 is titrated by addition of 0.100 M Na2CO3. 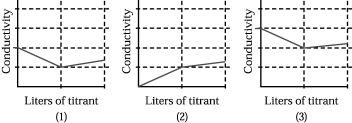
-Which of the above graphs corresponds to titration III?
Definitions:
Cost of Goods Sold
The direct costs attributable to the production of the goods sold by a company, including materials and labor.
Underapplied Overhead
When the actual manufacturing overhead costs are higher than the overhead allocated to products or services.
Actual Overhead Cost
The real expenses occurring from indirect costs, such as utilities, rent, and administrative expenses, necessary for running a business.
Indirect Labor Costs
Wages paid to employees who are not directly involved in production work but who support the production process.
Q2: Which one of the following contains 38.7%
Q7: Which element,indicated by letter on the periodic
Q23: The compound,NO<sub>2</sub>,is named<br>A)nitrate.<br>B)nitrite.<br>C)nitrogen dioxide.<br>D)nitrogen(IV)oxide.
Q27: Which molecule contains the most easily broken
Q65: Which electron affinity process would liberate the
Q66: To the nearest whole number,the molar mass
Q93: If the percent yield for the following
Q100: The mixing of which pair of reactants
Q164: Which elements commonly form anions?<br>A)A and B<br>B)A
Q198: A banana split is an example of<br>A)a