Multiple Choice
Box (1) represents 1.0 mL of a solution of particles at a given concentration. 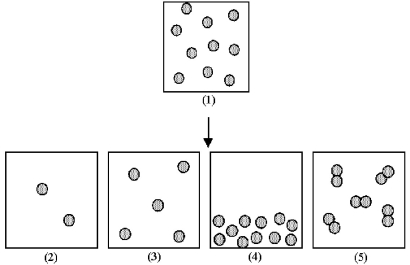
-Which of the boxes (2) -(5) represents 1.0 mL of the solution that results after (1) has been diluted by adding enough solvent to make 2.0 mL of solution?
Understand the legal and ethical considerations in making unilateral changes to employment terms.
Comprehend the challenges and benefits of integrative bargaining in collective bargaining processes.
Identify strategies for employers to engage with employees without violating the National Labor Relations Act.
Analyze the visible aspects of intraorganizational bargaining within unions compared to management.
Definitions:
Related Questions
Q13: How many electrons are in the outermost
Q31: The following diagram represents the reaction of
Q39: Assign formal charges to all atoms in
Q55: Of the following,which element has the highest
Q109: Photochemists use electromagnetic radiation to initiate chemical
Q137: Isoeugenol is the compound which gives the
Q146: Which of the following have the same
Q147: Predict the products of a reaction between
Q149: Which of the above fourth-shell orbitals is
Q159: If 200.mL of 0.100 M Na<sub>2</sub>SO<sub>4</sub> is