Multiple Choice
The four spheres below represent Na+,Mg2+,F⁻,and O2-,not necessarily in that order. 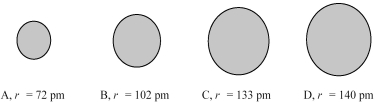
-Which sphere most likely represents the Mg2+ ion?
Identify the influence of external factors such as market conditions on strategic decisions in games.
Understand concepts like dominant strategy, Nash equilibrium, and their occurrence in various strategic settings.
Analyze strategic moves and counter-moves in sequential games.
Understand the effects of advertising and market competition on strategic decision-making in oligopolies.
Definitions:
Related Questions
Q6: Which of the following has the smallest
Q45: Which outcome corresponds to the combination of
Q68: What is the concentration of FeCl<sub>3</sub> in
Q70: How many lone pairs are on the
Q91: Which element can accommodate more than eight
Q119: Which molecular orbital resembles a p-orbital?<br>A)σ<br>B)σ<sup>*</sup><br>C)π<br>D)π<sup>*</sup>
Q121: Of the following,which element has the highest
Q129: What is the ground-state valence-shell electron configuration
Q144: Identify the set of hybrid orbitals shown
Q191: What is the oxidation number of the