Calculate the energy change in kJ/mol for the reaction 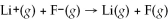 using the following information: Li(g) → Li+(g) + e- +520 kJ/mol
using the following information: Li(g) → Li+(g) + e- +520 kJ/mol
F(g) + e- → F-(g) -328 kJ/mol
Definitions:
Career
The pursuit of a series of related jobs over time in a particular area or industry, usually providing progression and personal growth.
Interpersonal Psychotherapists
are mental health professionals who specialize in a form of therapy focusing on interpersonal relationships and social functioning to treat depression and other disorders.
Psychoanalysis
Either the theory or the treatment of abnormal mental functioning that emphasizes unconscious psychological forces as the cause of psychopathology.
Bilateral Electroconvulsive Therapy
A form of electroconvulsive therapy in which electric currents are passed through the brain bilaterally (through both hemispheres) to induce a brief seizure, with the purpose of treating severe mental illnesses.
Q5: Classify bonds in As<sub>4</sub> as largely ionic,nonpolar
Q8: What are the possible values of n
Q40: Write a balanced net ionic equation for
Q60: What are the possible values of l
Q87: How many carbon-carbon bonds are present in
Q115: How many Fe(II)ions are there in 5.00
Q155: Because it forms some H<sup>+</sup> and OCl<sup>-</sup>
Q167: Which of the following should have the
Q176: What is the oxidation number of the
Q187: Which of the following compounds is not