Multiple Choice
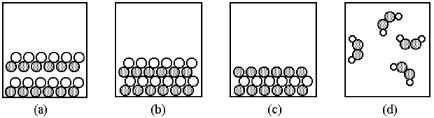
-Which of the above pictures are more likely to represent covalent compounds?
Explain the concepts of tariffs, quotas, and their impact on trade and exchange rates.
Connect fiscal policy changes to shifts in the macroeconomic indicators such as interest rates, net capital outflows, and exchange rates.
Understand the concepts of attributions and how they influence social perception and interaction.
Explain the difference between internal and external attributions and how they impact judgement.
Definitions:
Related Questions
Q22: The algebraic signs (+ and -)sometimes written
Q56: Which of the following exhibits ion-dipole forces?<br>A)KBr(s)<br>B)NaF(aq)<br>C)Ag(s)<br>D)Cl<sub>2</sub>(g)
Q112: When 0.700 g of anthracene,C<sub>14</sub>H<sub>10</sub>,is combusted in
Q124: The third-row element having a less negative
Q124: According to the Balmer-Rydberg equation,which transition results
Q138: Which has the highest Z<sub>eff</sub> for its
Q145: Which drawing best represents hydrogen bonding in
Q146: Which of the following have the same
Q148: How much heat is transferred per 5.00
Q165: For a hydrogen atom,which electronic transition would