Calculate the total quantity of heat required to convert 50.0 g of liquid CCl4(l) from 25.0°C to gaseous CCl4 at 76.8°C (the normal boiling point for CCl4) ? The specific heat of CCl4(l) is 0.857 J/(g ∙ °C) ,its heat of fusion is 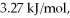 and its heat of vaporization is
and its heat of vaporization is 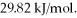
Definitions:
Experiment
A carefully controlled artificial situation that allows researchers to manipulate variables and measure the effects.
Field Research
The collection of raw data and information from real-life settings outside of a laboratory or structured environment.
Ethical Family Research
The practice of conducting research on family dynamics, structures, and relationships in a manner that respects the rights and dignity of participants.
Participant's Consent
A voluntary agreement by a person to participate in research, after being informed of the purpose, methods, risks, and benefits of the study.
Q9: Which sphere represents the metal atom?<br>A)A<br>B)B<br>C)C<br>D)D
Q16: Assign formal charges to each atom in
Q18: The following drawing is a representation of
Q23: If NO<sub>2</sub> and NH<sub>3</sub> are allowed to
Q97: Which ion has the same electron configuration
Q117: Bromine is one of only two elements
Q132: What is the geometry around the central
Q132: Which equation represents the reaction whose ΔH,represents
Q168: Is a hydroelectric dam a form of
Q202: What is the angle between adjacent sp<sup>3</sup>